
Frequently, by sensing tumors as an injury, the surrounding connective tissue is remodeled as in wound healing. This reaction is induced by molecules, such as the cytokine TGFβ, released by tumor or wound-injured epithelial cells acting on stromal fibroblasts and it is a trait of epithelial tumors located in soft organs, such as the breast, lung or intestine. As a result of this reaction, a peritumoral stroma rich in fibronectin and collagen fibers is generated. Tumor-associated demoplasia persists if the tumor does not remit, facilitating tumor cell mobility, invasion of adjacent tissues, and eventually intravasation into the blood or lymph vessels. Therefore, tumor demoplasia is associated with the formation of metastases. Our research goal is to understand this response of the tumor environment and the influence of the new chemical and physical properties on the formation of metastases.
We have described how healthy tumor-activated fibroblasts (CAFs) increase the composition, stiffness, and three-dimensional organization of extracellular fibers and how these parameters promote tumor invasion. At the molecular level, we have shown that the activity of a transcription factor called Snail1 analogously regulates the desmoplasia caused by CAFs, wound-activated fibroblasts, and fibroblasts activated in fibrotic processes. Snail1 is a transcriptional repressor but in these fibroblasts it can also act as a transcriptional activator and a splicing factor. We have defined some molecular elements, such as the RelA (p65) subunit of nuclear factor-kB (NF-kB), PARP1 and PRMTs1-4, which collaborate with Snail1 to increase the transcription of extracellular matrix molecules, such as Fibronectin. Snail1 is also required for the enzymatic activity of RhoA, which controls the reorganization of the cytoskeleton of fibroblasts and enables the polymerization of extracellular fibers, although the mechanism remains to be studied in detail.
When analyzing infiltrating breast carcinomas we have shown the relevance of Snail1 in tumor progression. The immuno-histochemical detection of Snail1 in fibroblasts of the tumor stroma accurately predicts poor prognosis. Of therapeutic interest, we have taken advantage of the data indicating that methyl transferases PRMT1 and 4 collaborate with Snail1 to reduce the formation of metastases by injecting methyl transferase inhibitors in a mouse cancer model (Figure 1).
With our current research we pursue deepening our knowledge on the molecular regulation of tumor-associated fibroblast. We are exploring into the actions of Snail1 on the extracellular matrix by controlling alternative splicing of structural molecules. We are therefore describing how splicing is regulated by Snail1 and which roles plays some splicing variants on the metastatic properties of matrices. We consider using specific splicing inhibitors based on modified nucleotides as anti-metastatic pharmacological tools, as this strategy is successfully used in pathologies associated with abnormal splicing variants.
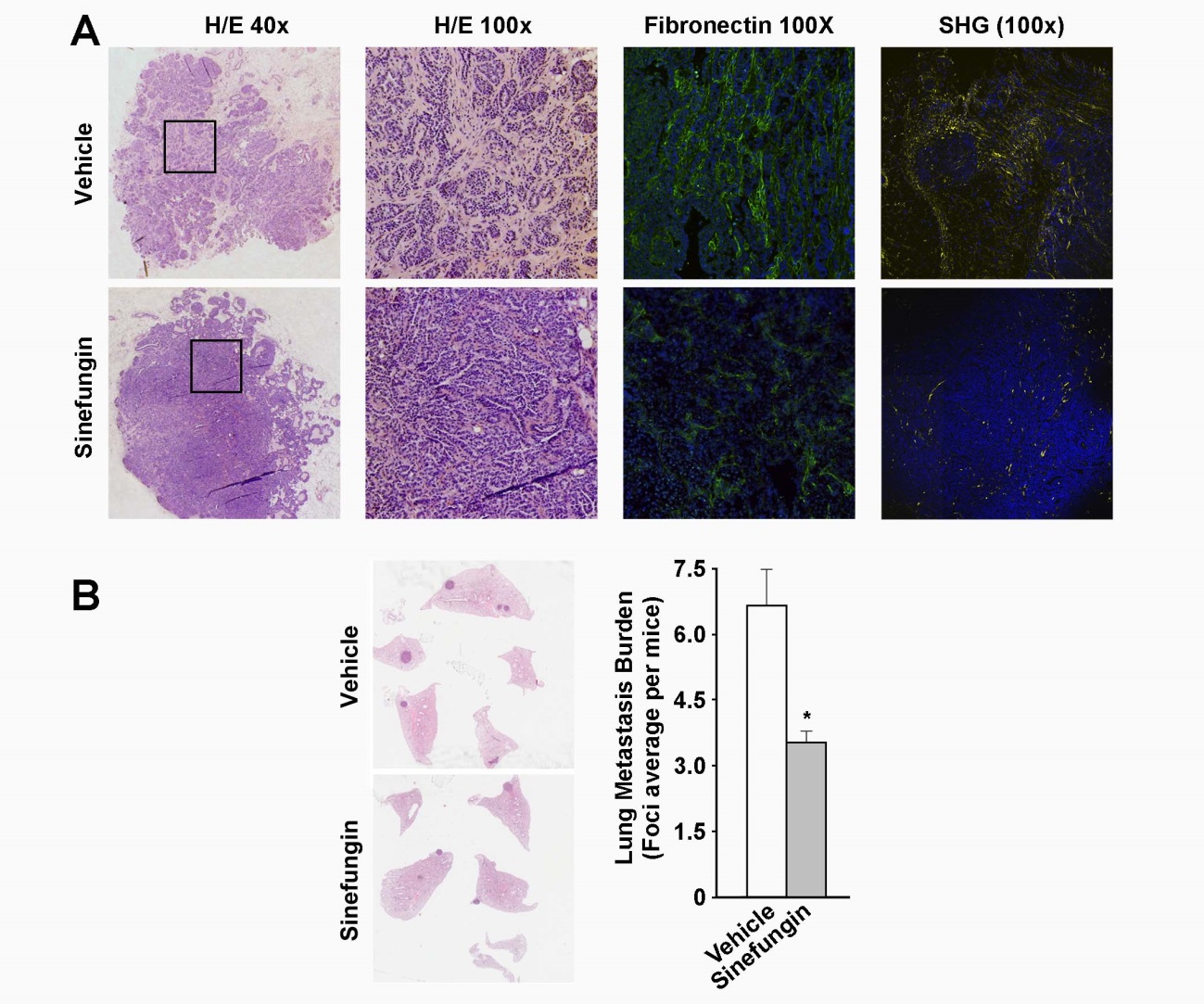
Figure 1. Sinefungin prevents stromal reaction and decreases metastasis in mice. (A) Primary breast tumors in mice treated or untreated with Sinefungin were analyzed by immuno-histochemistry (hematoxylin-eosin staining), immunofluorescence (fibronectin), or second harmonic generation (collagen). The stromal component of the tumors was reduced in treated mice. (B) Pulmonary metastatic foci were counted. The plot shows that Sinefungin treatment reduces lung metastasis burden. From Sala et al, 2019.
Four selected recent publications:
© Institut Hospital del Mar
d'Investigacions MèdiquesLegal Notice and Privacy Policy | Cookie Policy | Site Index | Accessibility | Find Us | Contact